Plasmids for SARS-CoV-2 Research
| Cat. No. | Plasmid Name | Description | Price | Ordering |
|---|---|---|---|---|
| MC_0101271 | SARS-CoV-2 Omicron Strain S gene original_pUC57 new | SARS-CoV-2 Omicron Strain | $420 | Add To Cart |
| MC_0101272 | SARS-CoV-2 Omicron Strain S gene Human codon_pUC57 new | SARS-CoV-2 Omicron Strain | $420 | Add To Cart |
| MC_0101273 | SARS-CoV-2 Omicron Strain S gene original_pcDNA3.1(+) new | SARS-CoV-2 Omicron Strain | $420 | Add To Cart |
| MC_0101274 | SARS-CoV-2 Omicron Strain S gene Human codon_pcDNA3.1(+) new | SARS-CoV-2 Omicron Strain | $420 | Add To Cart |
| MC_0101211 | pUC57-SARS-CoV-2-British Strain-S(Original) new | This plasmid contains the encoding gene of SARS-CoV-2 British Strain surface glycoprotein (original sequence) | $420 | Add To Cart |
| MC_0101212 | pUC57-SARS-CoV-2-British Strain-S(Human) new | This plasmid contains the encoding gene of SARS-CoV-2 British Strain surface glycoprotein (Codon optimized for Human expression system) | $420 | Add To Cart |
| MC_0101080 | pUC57-2019-nCoV-S(Original) | This plasmid contains the encoding gene of 2019-nCoV(SARS-CoV-2) surface glycoprotein (original sequence) | $420 | Add To Cart |
| MC_0101081 | pUC57-2019-nCoV-S(Human) | This plasmid contains the encoding gene of 2019-nCoV(SARS-CoV-2) surface glycoprotein (Codon optimized for Human expression system) | $420 | Add To Cart |
| MC_0101082 | pUC57-2019-nCoV-S(E. coli) | This plasmid contains the encoding gene of 2019-nCoV(SARS-CoV-2) surface glycoprotein (Codon optimized for E. Coli expression system) | $420 | Add To Cart |
| MC_0101083 | pUC57-2019-nCoV-S(CHO) | This plasmid contains the encoding gene of 2019-nCoV(SARS-CoV-2) surface glycoprotein (Codon optimized for CHO expression system) | $420 | Add To Cart |
| MC_0101084 | pUC57-2019-nCoV-S(Insect) | This plasmid contains the encoding gene of 2019-nCoV(SARS-CoV-2) surface glycoprotein (Codon optimized for Insect expression system) | $420 | Add To Cart |
| MC_0101129 | pcDNA3.1(+)-P2A-eGFP-ORF10 | The plasmid is used for SARS-CoV-2 ORF10 protein expression. The ORF10 protein is integrated with eGFP protein through P2A, which can be used for flow cytometry antibody screening or immunological research. | $140 | Add To Cart |
| MC_0101130 | pcDNA3.1(+)-P2A-eGFP-ORF8 | The plasmid is used for SARS-CoV-2 ORF8 protein expression. The ORF8 protein is integrated with eGFP protein through P2A, which can be used for flow cytometry antibody screening or immunological research. | $140 | Add To Cart |
| MC_0101131 | pcDNA3.1(+)-P2A-eGFP-ORF6 | The plasmid is used for SARS-CoV-2 ORF6 protein expression. The ORF6 protein is integrated with eGFP protein through P2A, which can be used for flow cytometry antibody screening or immunological research. | $140 | Add To Cart |
| MC_0101132 | pcDNA3.1(+)-P2A-eGFP-ORF3a | The plasmid is used for SARS-CoV-2 ORF3a protein expression. The ORF3a protein is integrated with eGFP protein through P2A, which can be used for flow cytometry antibody screening or immunological research. | $140 | Add To Cart |
| MC_0101133 | pcDNA3.1(+)-P2A-eGFP-ORF7a | The plasmid is used for SARS-CoV-2 ORF7a protein expression. The ORF7a protein is integrated with eGFP protein through P2A, which can be used for flow cytometry antibody screening or immunological research. | $140 | Add To Cart |
| MC_0101134 | pcDNA3.1(+)-P2A-eGFP-ORF7b | The plasmid is used for SARS-CoV-2 ORF7b protein expression. The ORF7b protein is integrated with eGFP protein through P2A, which can be used for flow cytometry antibody screening or immunological research. | $140 | Add To Cart |
| MC_0101135 | pcDNA3.1(+)-P2A-eGFP-E Protein | The plasmid is used for SARS-CoV-2 envelope protein protein expression. The envelope protein n is integrated with eGFP protein through P2A, which can be used for flow cytometry antibody screening or immunological research. | $140 | Add To Cart |
| MC_0101136 | pET-28a(+)-M Protein | The plasmid is used for SARS-CoV-2 membrane glycoprotein expression. | $140 | Add To Cart |
| MC_0101137 | pcDNA3.1(+)-N-eGFP-N Protein | The plasmid is used for SARS-CoV-2 nucleocapsidphos phoprotein expression. | $140 | Add To Cart |
| MC_0101076 | pUC57-2019-nCoV-PC:RdRP | This plasmid contains part of 2019-nCoV(SARS-CoV-2) RdRP gene and can be used as positive control for the detection of 2019-nCoV(SARS-CoV-2) by qRT-PCR | $85 | Add To Cart |
| MC_0101077 | pUC57-2019-nCoV-PC:N | This plasmid contains part of 2019-nCoV(SARS-CoV-2) N gene and can be used as positive control for the detection of 2019-nCoV(SARS-CoV-2) by qRT-PCR | $85 | Add To Cart |
| MC_0101078 | pUC57-2019-nCoV-PC:E | This plasmid contains part of 2019-nCoV(SARS-CoV-2) E gene and can be used as positive control for the detection of 2019-nCoV(SARS-CoV-2) by qRT-PCR | $85 | Add To Cart |
| MC_0101079 | pUC57-2019-nCoV-PC:ORF1ab | This plasmid contains part of 2019-nCoV(SARS-CoV-2) ORF1ab and can be used as positive control for the detection of 2019-nCoV(SARS-CoV-2) by qRT-PCR | $85 | Add To Cart |
| MC_0101085 | pUC57-2019-nCoV-N | This plasmid contains the encoding gene of 2019-nCoV(SARS-CoV-2) nucleocapsid phosphoprotein (original sequence) | $140 | Add To Cart |
| MC_0101086 | pcDNA3.1+/C-(K)DYK-ACE2 (NM_021804.2, OHu20260) 30% off | Homo sapiens angiotensin I converting enzyme 2 (ACE2), the receptor for 2019-nCoV(SARS-CoV-2). | $379 30% off | Add To Cart |
Plasmids Used for SARS-CoV-2 Research Are Shared by Cloud Scientist
| Cat. No. | Plasmid Name | Description | Price | Ordering |
|---|---|---|---|---|
| MC_0101087 | 2019-nCov_pcDNA3.1(+)-P2A-eGFP | The plasmid is used for 2019-nCoV(SARS-CoV-2) surface glycoprotein expression (Codon Optimized for Mouse expression system). The surface glycoprotein is integrated with eGFP protein through P2A, which can be used for flow cytometry antibody screening or immunological research. | $65 | Add To Cart |
| MC_0101088 | SARS_pcDNA3.1(+)-P2A-eGFP | The plasmid is used for SARS surface glycoprotein expression (Codon Optimized for Mouse expression system). The surface glycoprotein is integrated with eGFP protein through P2A, which can be used for flow cytometry antibody screening or immunological research. | $65 | Add To Cart |
| MC_0101089 | 2019-nCov-Linker_pcDNA3.1(+)-C-eGFP | The plasmid is used for 2019-nCoV(SARS-CoV-2) surface glycoprotein expression (Codon Optimized for Mouse expression system). The surface glycoprotein is integrated with eGFP protein through linker, which can be used for flow cytometry antibody screening. | $65 | Add To Cart |
| MC_0101090 | SARS-Linker_pcDNA3.1(+)-C-eGFP | The plasmid is used for SARS surface glycoprotein expression (Codon Optimized for Mouse expression system). The surface glycoprotein is integrated with eGFP protein through linker, which can be used for flow cytometry antibody screening. | $65 | Add To Cart |
Customized Protein Service and Products for SARS-CoV-2 Research
| Protein Name | System | Size | List Price (USD) | Cat.No. | Timeline | Ordering |
|---|---|---|---|---|---|---|
| SARS-CoV-2 Spike protein (RBD, His Tag) | Sf9 insect cells | 1mg | $2,100 | Z03479-1 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (RBD, His Tag) | Sf9 insect cells | 100μg | $380 | Z03479-100 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (RBD, His Tag) | Sf9 insect cells | 500μg | $1,200 | Z03479-500 | In stock | Quick Order |
| SARS-CoV-2 Nucleocapsid protein (His Tag) | E.coli | 1mg | $2,100 | Z03480-1 | In stock | Quick Order |
| SARS-CoV-2 Nucleocapsid protein (His Tag) | E.coli | 100μg | $380 | Z03480-100 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (ECD, His & Flag Tag) | Sf9 insect cells | 1mg | $2,100 | Z03481-1 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (ECD, His & Flag Tag) | Sf9 insect cells | 100μg | $380 | Z03481-100 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (RBD, His Tag) | Human Cells | 1mg | $2,100 | Z03483-1 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (RBD, His Tag) | Human Cells | 100μg | $380 | Z03483-100 | In stock | Quick Order |
| ACE-2 Fc Chimera, Human | Human Cells | 1mg | $2,100 | Z03484-1 | In stock | Quick Order |
| ACE-2 Fc Chimera, Human | Human Cells | 100μg | $380 | Z03484-100 | In stock | Quick Order |
| SARS-CoV-2 S1 protein(His-tagged) | Human Cells | 1mg | $2,100 | Z03485-1 | In stock | Quick Order |
| SARS-CoV-2 S1 protein(His-tagged) | Human Cells | 100μg | $380 | Z03485-100 | In stock | Quick Order |
| SARS-CoV-2 Nucleocapsid protein | E.coli | 1mg | $2,100 | Z03488-1 | In stock | Quick Order |
| SARS-CoV-2 Nucleocapsid protein | E.coli | 100μg | $380 | Z03488-100 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (RBD, mFC Tag) | Human Cells | 1mg | $2,100 | Z03491-1 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (RBD, mFC Tag) | Human Cells | 100μg | $380 | Z03491-100 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (S1) | Human Cells, tag-free | 1mg | $2,100 | Z03501-1 | In stock | Quick Order |
| SARS-CoV-2 Spike protein (S1) | Human Cells, tag-free | 100μg | $380 | Z03501-100 | In stock | Quick Order |
| 2019-nCoV N Protein (His-tagged) | Insect | 0.5mg | $1,000 | Customized service | 2 weeks | Please send your request to protein@genscript.com |
| 1mg | $1,500 | Customized service | 2 weeks | |||
| 1.5mg | $2,000 | Customized service | 2 weeks | |||
| 2mg | $2,500 | Customized service | 2 weeks | |||
| Functional 2019-nCoV S protein (RBD-His) | Insect | 0.5mg | $1,000 | Customized service | 2 weeks | |
| 1mg | $1,500 | Customized service | 2 weeks | |||
| 1.5mg | $2,000 | Customized service | 2 weeks | |||
| 2mg | $2,500 | Customized service | 2 weeks | |||
| Functional 2019-nCoV S protein (RBD-mFc) | Mammalian | 0.5mg | $1,000 | Customized service | 2 weeks | |
| 1mg | $1,500 | Customized service | 2 weeks | |||
| 1.5mg | $2,000 | Customized service | 2 weeks | |||
| 2mg | $2,500 | Customized service | 2 weeks | |||
| Functional 2019-nCoV S protein (RBD) | Mammalian | / | / | Customized service | 2 weeks | |
| 2019-nCoV S protein (ECD His-tagged) | Insect & Mammalian | / | / | Customized service | 2 weeks |
* 2019-nCoV is also also known as SARS-CoV-2.
** The prices are for MolecularCloud customers exclusively.
*** For customized services, the timeline is estimated and excluded shipping time.
**** For other reagent protein services, please send your request to protein@genscript.com. Our service representative will contact you with a quote shortly.
Custom peptide and peptide library services for SARS-CoV-2 Research
All of Peptide libraries are designed by overlapping peptide library tool
| Protein Name | Source | Number of peptide | Purity | Sequence | Timeline | Ordering |
|---|---|---|---|---|---|---|
| SARS-CoV-2 Spike Glycoprotein B.1.1.529 (Omicron Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein B.1.1.529 (Omicron Variant) | 315 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks(Crude) | Please send your request to peptide@genscript.com Find more peptide services |
|
| SARS-CoV-2 Spike Glycoprotein B.1.617.2 (Delta Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein B.1.617.2 (Delta Variant) | 315 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks(Crude) | ||
| SARS-CoV-2 Spike Glycoprotein B.1.617.1 (Kappa Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein B.1.617.1 (Kappa Variant) | 316 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 4weeks(Crude) | ||
| SARS-CoV-2 Spike Glycoprotein RBD B.1.617.1 (Kappa Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein RBD B.1.617.1 (Kappa Variant) | 53 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks(Crude) | ||
| SARS-CoV-2 Spike Glycoprotein B.1.1.7 (Alpha Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein B.1.1.7 (Alpha Variant) | 315 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 4weeks(Crude) | ||
| SARS-CoV-2 Spike Glycoprotein RBD B.1.1.7 (Alpha Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein RBD B.1.1.7 (Alpha Variant) | 53 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks(Crude) | ||
| SARS-CoV-2 Spike Glycoprotein B.1.351 (Beta Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein B.1.351 (Beta Variant) | 315 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 4weeks(Crude) | ||
| SARS-CoV-2 Spike Glycoprotein RBD B.1.351 (Beta Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein RBD B.1.351 (Beta Variant) | 53 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks(Crude) | ||
| SARS-CoV-2 Spike Glycoprotein P.1 (Gamma Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein P.1 (Gamma Variant) | 316 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 4weeks(Crude) | ||
| SARS-CoV-2 Spike Glycoprotein RBD P.1 (Gamma Variant)new | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein RBD P.1 (Gamma Variant) | 53 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks(Crude) | ||
| HCoV-229E Spike Glycoprotein | Human coronavirus 229E (HCoV-229E) Spike glycoprotein | 291 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 4weeks(Crude) | ||
| HCoV-OC43 Spike glycoprotein | Human coronavirus OC43 (HCoV-OC43) Spike glycoprotein | 336 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 4weeks(Crude) | ||
| SARS-CoV-2 ORF3a | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Protein 3a | 66 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks(Crude) | ||
| SARS-CoV-2 N | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Nucleoprotein | 102 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks(Crude) | ||
| SARS-CoV-2 ORF6 | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Non-structural protein 6 | 13 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 2weeks(Crude) | ||
| SARS-CoV-2 ORF7b | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Non-structural Protein 7b | 8 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 2weeks(Crude) | ||
| SARS-CoV-2 ORF7a | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Non-structural protein 7A | 28 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 2weeks(Crude) | ||
| SARS-CoV-2 ORF8 | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Non-structural protein 8 | 28 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 2weeks(Crude) | ||
| SARS-CoV-2 ORF10 | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) ORF10 Protein | 7 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 2weeks(Crude) | ||
| SARS-CoV-2 ORF9B | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Protein 9b | 22 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 2weeks(Crude) | ||
| SARS-CoV-2 Spike Glycoprotein | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Spike glycoprotein | 316 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 4weeks(Crude) | ||
| SARS-CoV-2 E | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Envelope small membrane protein | 16 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 2weeks(Crude) | ||
| SARS-CoV-2 M | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Membrane protein | 53 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks | ||
| SARS-CoV-2 Y14 | SARSr-CoV (Severe Acute Respiratory Syndrome-related coronavirus 2) Uncharacterized protein 14 | 16 peptides (15mers with 11 aa overlap) | Crude, ≥70% to ≥98% | 3weeks |
The following table shows the most popular SARS-CoV-2 peptides published on journals
| Cat. No. | Peptide Name | Description | Ordering |
|---|---|---|---|
| MCP_0001634 New | SARS-CoV-2 Spike glycoprotein, 647-664 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide AGCLIGAEHVNNSYECDI for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001633 New | SARS-CoV-2 Spike glycoprotein, 747-763 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide TECSNLLLQYGSFCTQL, probable allele specificity HLA-DR8, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001632 New | SARS-CoV-2 Spike glycoprotein, 691-699 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide SIIAYTMSL, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001631 New | SARS-CoV-2 Spike glycoprotein, 424-433 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide KLPDDFTGCV, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001630 New | SARS-CoV-2 Spike glycoprotein, 996-1004 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide LITGRLQSL, probable allele specificity HLA-A2;HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001629 New | SARS-CoV-2 Spike glycoprotein, 976-984 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide VLNDILSRL, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001628 New | SARS-CoV-2 Spike glycoprotein, 958-966 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide ALNTLVKQL, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001627 New | SARS-CoV-2 Spike glycoprotein, 957-973 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen PeptideQALNTLVKQLSSNFGAI, probable allele specificity HLA-DRB1*04:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001626 New | SARS-CoV-2 Spike glycoprotein, 902-917 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide MAYRFNGIGVTQNVLY, probable allele specificity HLA-DRB1*04:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001625 New | SARS-CoV-2 Spike glycoprotein, 891-906 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide GAALQIPFAMQMAYRF, probable allele specificity HLA-DRA*01:01/DRB1*07:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001624 New | SARS-CoV-2 Spike glycoprotein, 1220-1228 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide FIAGLIAIV, probable allele specificity HLA-A2;HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001623 New | SARS-CoV-2 Spike glycoprotein, 1192-1200 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide NLNESLIDL, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001622 New | SARS-CoV-2 Spike glycoprotein, 1185-1193 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide RLNEVAKNL, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001621 New | SARS-CoV-2 Spike glycoprotein, 1060-1068 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide VVFLHVTYV, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001620 New | SARS-CoV-2 Spike glycoprotein, 1011-1028 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Spike glycoprotein (Uniprot No: P0DTC2). Antigen Peptide QLIRAAEIRASANLAATK, probable allele specificity HLA-DRB1*04:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001619 New | SARS-CoV-2 Membrane protein, 89-97 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Membrane protein (Uniprot No: P0DTC5). Antigen Peptide GLMWLSYFI, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001618 New | SARS-CoV-2 Membrane protein, 61-70 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Membrane protein (Uniprot No: P0DTC5). Antigen Peptide TLACFVLAAV, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001617 New | SARS-CoV-2 Nucleoprotein, 329-353 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide TWLTYTGAIKLDDKDPNFKDQVILL for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001616 New | SARS-CoV-2 Nucleoprotein, 329-346 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide TWLTYTGAIKLDDKDPNF for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001615 New | SARS-CoV-2 Nucleoprotein, 215-229 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide GDAALALLLLDRLNQ for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001614 New | SARS-CoV-2 Nucleoprotein, 125-139 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide ANKDGIIWVATEGAL, probable allele specificity HLA class II, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001613 New | SARS-CoV-2 Nucleoprotein, 325-339 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide TPSGTWLTYTGAIKL, probable allele specificity HLA class II, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001612 New | SARS-CoV-2 Nucleoprotein, 320-334 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide IGMEVTPSGTWLTYT, probable allele specificity HLA class I, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001611 New | SARS-CoV-2 Nucleoprotein, 265-274 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide TKAYNVTQAF, probable allele specificity HLA class I, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001610 New | SARS-CoV-2 Nucleoprotein, 260-274 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide QKRTATKAYNVTQAF, probable allele specificity HLA class I;HLA-B*15:25, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001609 New | SARS-CoV-2 Nucleoprotein, 226-234 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide RLNQLESKM, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001608 New | SARS-CoV-2 Nucleoprotein, 40-54 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide RRPQGLPNNTASWFT, probable allele specificity HLA class I, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001607 New | SARS-CoV-2 Nucleoprotein, 360-375 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide YKTFPPTEPKKDKKKK for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001606 New | SARS-CoV-2 Nucleoprotein, 352-369 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide LLNKHIDAYKTFPPTEPK for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001605 New | SARS-CoV-2 Nucleoprotein, 351-358 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide ILLNKHID, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001604 New | SARS-CoV-2 Nucleoprotein, 351-359 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide ILLNKHIDA, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001603 New | SARS-CoV-2 Nucleoprotein, 322-331 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide MEVTPSGTWL, probable allele specificity HLA-B*40:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001602 New | SARS-CoV-2 Nucleoprotein, 316-324 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide GMSRIGMEV, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001601 New | SARS-CoV-2 Nucleoprotein, 313-330 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide SARS-CoV-2 NCAP (AFFGMSRIGMEVTPSGTW) for stimulation of antigen-specific T cells in T cell assays such as ELISPOT, ICS, cytotoxity or proliferation assays. | Quick Order |
| MCP_0001600 New | SARS-CoV-2 Nucleoprotein, 305-319 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide AQFAPSASAFFGMSR, probable allele specificity HLA class II, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001599 New | SARS-CoV-2 Nucleoprotein, 305-322 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide AQFAPSASAFFGMSRIGM is used for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001598 New | SARS-CoV-2 Nucleoprotein,299-315 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide KHWPQIAQFAPSASAFFis used for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001597 New | SARS-CoV-2 Nucleoprotein, 292-308 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide IRQGTDYKHWPQIAQFA is used for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001596 New | SARS-CoV-2 Nucleoprotein, 222-230 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide LLLDRLNQL, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001595 New | SARS-CoV-2 Nucleoprotein, 219-227 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide ILLNKHIDA, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays. | Quick Order |
| MCP_0001594 New | SARS-CoV-2 Nucleoprotein, 159-167 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide LQLPQGTTL, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays | Quick Order |
| MCP_0001593 New | SARS-CoV-2 Nucleoprotein, 138-146 | This peptide is derived from severe acute respiratory syndrome coronavirus 2(SARS-Cov-2), Nucleoprotein(Uniprot No: P0DTC9). Antigen Peptide ALNTPKDHI, probable allele specificity HLA-A*02:01, for stimulation of antigen-specific T cells in T cell assays | Quick Order |
| MCP_0001569 | (Biotin - Ahx) - SARS - CoV - 2 Spike RBM (receptor binding motif) | This peptide sequence has residues 438-458 obtained from the RBM (receptor binding motif: 437-508) identified within the RBD from the RefSeq (YP_009724390.1) of spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the N-terminus. | Quick Order |
| MCP_0001570 | SARS - CoV - 2 Spike RBD (receptor binding domain), 319 - 335 | This peptide sequence represents residues 319-335 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001571 | SARS - CoV - 2 Spike RBD (receptor binding domain), 319 - 335 - Lys(Biotin - Ahx) | This peptide sequence represents residues 319-335 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001572 | SARS - CoV - 2 Spike RBD (receptor binding domain), 336 - 347 | This peptide sequence represents residues 336-347 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001573 | SARS - CoV - 2 Spike RBD (receptor binding domain), 336 - 347 - Lys(Biotin - Ahx) | This peptide sequence represents residues 336-347 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001574 | SARS - CoV - 2 Spike RBD (receptor binding domain), 348 - 357 | This peptide sequence represents residues 348-357 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001575 | SARS - CoV - 2 Spike RBD (receptor binding domain), 348 - 357 - Lys(Biotin - Ahx) | This peptide sequence represents residues 348-357 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001576 | SARS - CoV - 2 Spike RBD (receptor binding domain), 352 - 365 | This peptide sequence represents residues 352-365 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001577 | SARS - CoV - 2 Spike RBD (receptor binding domain), 352 - 365 - Lys(Biotin - Ahx) | This peptide sequence represents residues 352-365 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001578 | SARS - CoV - 2 Spike RBD (receptor binding domain), 371 - 394 | This peptide sequence represents residues 371-394 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001579 | SARS - CoV - 2 Spike RBD (receptor binding domain), 371 - 394 - Lys(Biotin - Ahx) | This peptide sequence represents residues 371-394 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001580 | SARS - CoV - 2 Spike RBD (receptor binding domain), 395 - 430 | This peptide sequence represents residues 395-430 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001581 | SARS - CoV - 2 Spike RBD (receptor binding domain), 395 - 430 - Lys(Biotin - Ahx) | This peptide sequence represents residues 395-430 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001582 | SARS - CoV - 2 Spike RBD (receptor binding domain), 513 - 520 | This peptide sequence represents residues 513-520 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2 | Quick Order |
| MCP_0001583 | SARS - CoV - 2 Spike RBD (receptor binding domain), 513 - 520 - Lys(Biotin - Ahx) | This peptide sequence represents residues 513-520 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001584 | SARS - CoV - 2 Spike RBD (receptor binding domain), 523 - 541 | This peptide sequence represents residues 523-541 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001585 | SARS - CoV - 2 Spike RBD (receptor binding domain), 523 - 541 - Lys(Biotin - Ahx) | This peptide sequence represents residues 523-541 of the RBD (receptor binding domain) identified from the RefSeq (YP_009724390.1) from the spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001586 | SARS - CoV - 2 Spike RBM (receptor binding motif), 438 - 458 | This peptide sequence has residues 438-458 obtained from the RBM (receptor binding motif: 437-508) identified within the RBD from the RefSeq (YP_009724390.1) of spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001587 | SARS - CoV - 2 Spike RBM (receptor binding motif), 450 - 473 | This peptide sequence has residues 450-473 obtained from the RBM (receptor binding motif: 437-508) identified within the RBD from the RefSeq (YP_009724390.1) of spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001588 | SARS - CoV - 2 Spike RBM (receptor binding motif), 450 - 473 - Lys(Biotin - Ahx) | This peptide sequence has residues 450-473 obtained from the RBM (receptor binding motif: 437-508) identified within the RBD from the RefSeq (YP_009724390.1) of spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001589 | SARS - CoV - 2 Spike RBM (receptor binding motif), 480 - 496 | This peptide sequence has residues 480-496 obtained from the RBM (receptor binding motif: 437-508) identified within the RBD from the RefSeq (YP_009724390.1) of spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001590 | SARS - CoV - 2 Spike RBM (receptor binding motif), 480 - 496 - Lys(Biotin - Ahx) | This peptide sequence has residues 480-496 obtained from the RBM (receptor binding motif: 437-508) identified within the RBD from the RefSeq (YP_009724390.1) of spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0001591 | SARS - CoV - 2 Spike RBM (receptor binding motif), 500 - 509 | This peptide sequence has residues 500-509 obtained from the RBM (receptor binding motif: 437-508) identified within the RBD from the RefSeq (YP_009724390.1) of spike protein of SARS-CoV-2. | Quick Order |
| MCP_0001592 | SARS - CoV - 2 Spike RBM (receptor binding motif), 500 - 509 - Lys(Biotin - Ahx) | This peptide sequence has residues 500-509 obtained from the RBM (receptor binding motif: 437-508) identified within the RBD from the RefSeq (YP_009724390.1) of spike protein of SARS-CoV-2. The sequence is labeled with Biotin-Ahx at the C-terminus. | Quick Order |
| MCP_0000053 | Angiotensin I Converting Enzyme 2, (ACE - 2) Substrate | An ACE-2 (Angiotensin I-converting enzyme 2) fluorescent substrate. Complete hydrolysis of 0.04 mM results in a 300-fold fluorescence increase over background. Max Abs/Em=325/393 nm upon cleavage of substrate. | Quick Order |
| MCP_0000359 | DX 600, ACE2 Inhibitor | A potent inhibitor specific for ACE2. It has a Ki of 2.8 nM. | Quick Order |
|
|
|||
2019-nCoV qRT-PCR Detection Assay
How to detect the SARS-CoV-2 virus?
Principle of novel coronavirus detection
The virus detection method can be basically illustrated as below.
Step 1:
Isolate the novel coronavirus (2019-nCoV) from the patients and sequence its genome.
Step 2:
Compare the genome sequence of 2019-nCoV with human genome to find out the specific sequence in the virus genome.
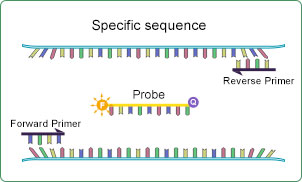
Step 3:
Design PCR amplification primers and fluorescent probe primers for detecting the specific sequences identified in step 2.
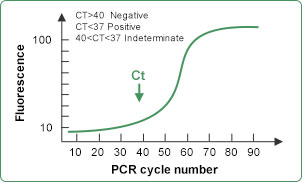
Step 4:
Extract RNA from suspected individual’s serum, and convert the RNA into cDNA. The cDNA is then used as template and mixed with the PCR primers and probes for amplification. If the fluorescence signal increases rapidly and Ct value is less than 37, it can be determined as positive; If there’s no fluorescence detected, or the fluorescence signal grows slowly and Ct value finally ends up above 40, it can be determined as negative.
Resources
WHO guidanceOn 17 January 2020, WHO provided interim guidance to laboratories and stakeholders involved in laboratory testing of patients who meet suspected case of pneumonia associated with the 2019 novel coronavirus.
Available protocols- Diagnostic detection of Wuhan coronavirus 2019 by real-time RT-PCR – Charité, Berlin Germany (pdf)
- Detection of 2019 novel coronavirus (2019-nCoV) in suspected human cases by RT-PCR – Hong Kong University (pdf)
- China CDC Primers and probes for detection 2019-nCoV
- SARS-CoV-2 qRT-PCR detection assay-1 step-1 plex Manual (pdf)
- GenScript COVID-19 detection tech notes (pdf)