Neoantigen, also called tumor-specific antigen (TSA), is a repertoire of peptides that arise as the result of DNA mutations in tumor cells. Unlike the other two common types of tumor antigens, named tumor-associated antigens (TAAs) and cancer-germline antigens (CGAs), tumor neoantigen is the truly foreign protein and entirely absent from normal human organs or tissues. As a result, targeting neoantigens could allow the patient's immune system to exclusively find and attack cancer cells without harming normal healthy cells. Decades of work has increasingly shown their potential for cancer immunotherapy.
In this article, we will systematically introduce the history of understanding and identification of neoantigens, how to develop personalized neoantigen-based vaccines, how neoantigen vaccines work, their role on current cancer immunotherapies and the key players in the industry.
Genetic instability of tumor cells often leads to a large number of mutations, and expression of non-synonymous mutations can produce tumor-specific antigens, called neoantigens [1]. Some of the neoantigens can be expressed, processed and presented on the cell surface, and subsequently recognized by T cells in the context of major histocompatibility complexes (MHCs) molecules. Because neoantigens are not expressed in normal tissues, neoantigen-specific T cells are not subject to central and peripheral tolerance, and also lack the ability to induce normal tissue destruction. As a result, neoantigens appear to be the ideal targets for T cell-based cancer immunotherapy [2].
How are neoantigens discovered?
In the early twentieth century, many findings revealed that the immune system can recognize and eliminate tumor cells. However, the nature of antigens that could trigger antitumor immune response was unclear during that time. Until 1988 De Plaen and colleagues identified the first neoantigen that can be recognized by T cells in a mouse tumor model by using cDNA library screening [3, 4]. They found that only one nucleotide differed between the normal and tumor gene and this mutation produced an amino acid change. After that, a series of neoantigens derived from somatic mutations were identified in various human tumors including melanoma and renal cell carcinoma [3].
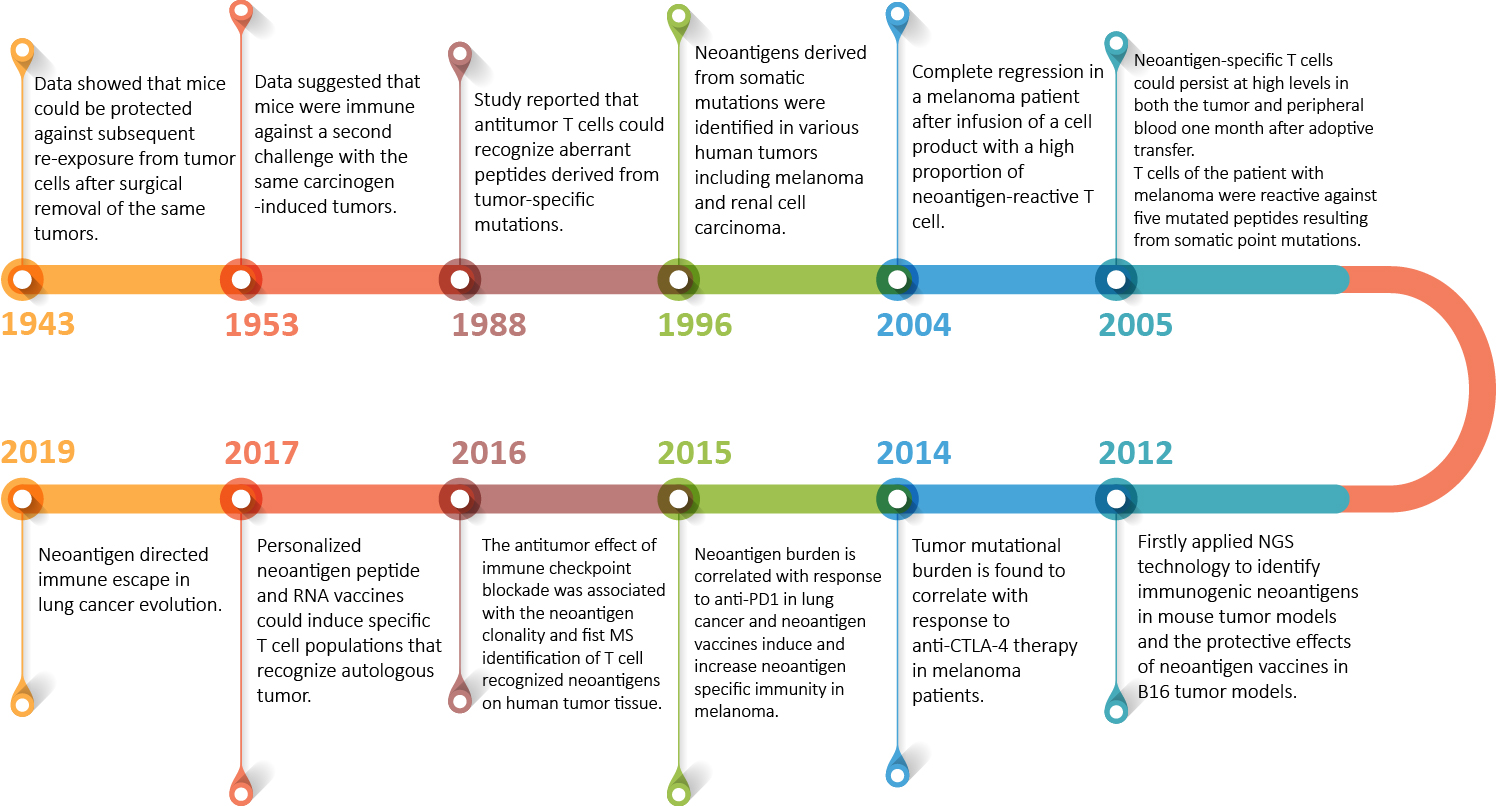
Fig.1 Historical overview of tumor neoantigens. The figure is originally published by Tao Jiang et al [3] and designed by MolecularCloud.
How to identify neoantigens in tumor cells?
According to articles published during 1995-2013, most of the neoantigens were identified by cDNA library screening. In this approach, cDNA library and MHC molecules were over-expressed in cell lines, and then co-cultured with T cells to identify antigens that could induce the T cell activation [2]. However, this method is labor-intensive, expensive and cannot effectively identify all tumor antigens. The rapid development of next-generation sequencing (NGS) technology makes it possible to rapidly compare the DNA sequences of tumor cells and normal cells and identify tumor-specific mutations. In 2012, NGS technology was firstly reported to be applied in identifying neoantigens in mouse tumor models [5, 6] and soon widely used. Currently, based on the whole exome sequencing (WES) technology and constantly optimized bioinformatics algorithms, a lot of neoantigens have been identified with high efficiency, wide coverage, and low false negative rate [1]. Even though sophisticated machine learning methods are used to winnow down candidate numbers returned by prediction algorithms, scientists have to go back to wet lab experiments to validate that the potential neoantigens are active and could be able to trigger bona fide antitumor responses in patients [7].
Step 1: Identification of tumor non-synonymous mutations (NSM). WES is performed on tumor and normal DNA to identify tumor-specific NSM. When available, RNA-seq is used to select mutations that are expressed.
Step 2: Selection of candidate neoantigens. Once NSMs are identified, three strategies can be used to select the list of candidate neoantigens that will be assessed for immunogenicity.
Step 3: Evaluation of immunogenicity of candidate neoantigens. Finally, the immunogenicity of the selected candidate peptides is evaluated with different immunological screening assays.
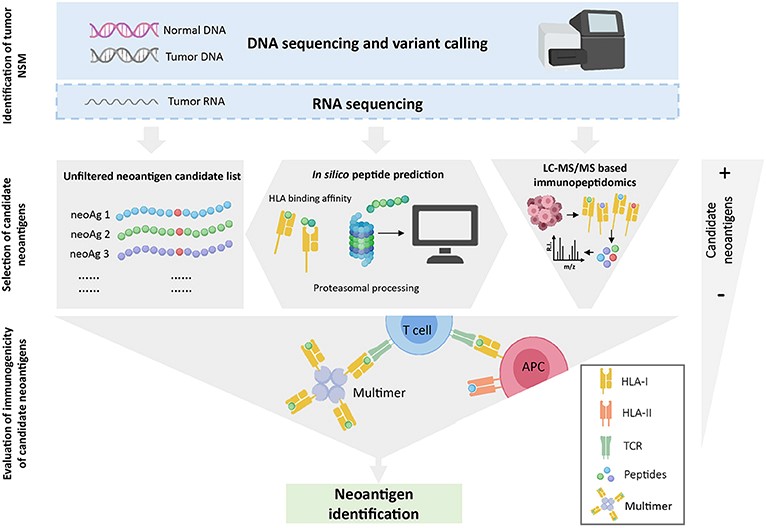
Fig.2 Overview of neoantigen
identification using tumor WES [8].
Common prophylactic vaccines introduce antigens into a person's body to make immune system generate antibodies for those antigens, and become immune to the associated illness. While tumor vaccines are administered to patients with malignant tumors to activate the patient's autoimmune response and kill the tumor cells. Traditional tumor vaccines mainly target tumor-associated antigens (TAAs), which are existing in both tumor cells and normal cells. Several clinical trials targeting TAAs with anti-tumor vaccines have failed to achieve the desired therapeutic effect. Neoantigens, differed from the traditional TAAs, are tumor-specific antigens. Compared to TAAs, neoantigens possess stronger immunogenicity and higher affinity toward MHC, and are not affected by central immunological tolerance. Therefore designing specific neoantigens-based vaccines would be a better solution for tumor immunotherapy. The first human clinical trial using neoantigen vaccines was reported in 2015 by Carreno and colleagues. In their study, they vaccinated three patients with advanced melanoma with personalized dendritic cell-based vaccines designed to activate T cells specific for mutations in the patients' cancer and indeed expanded the T cells immunity and breadth of the antitumor immune response [9].
Major types of neoantigen vaccine
Tumor vaccines targeting neoantigens mainly include nucleic acid, dendritic cell (DC)-based, tumor cell, and synthetic long peptide (SLP) vaccines [1]. (Fig. 3)
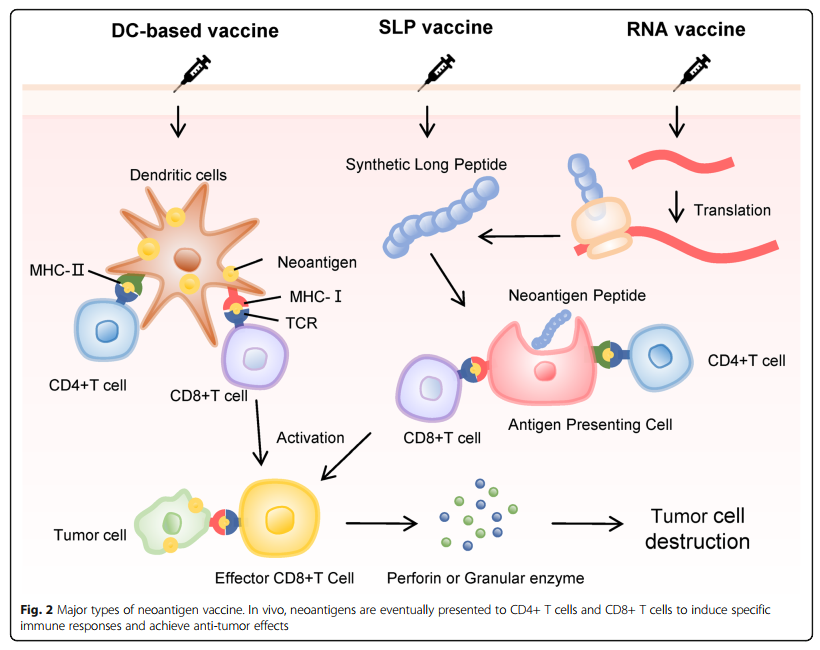
Fig.3 Major types of neoantigen vaccines. In vivo, neoantigens are eventually presented to CD4+ T cells and CD8+
T cells to induce specific immune responses and achieve anti-tumor effects [1].
Each method has distinct advantages and disadvantages. In brief, dendritic cell (DC)-based vaccines have low toxicity, but are not very potent and require additional steps to have antigens loaded and presented. The RNA neoantigen vaccine has unique advantages. It only requires a small amount of tumor cells to extract RNA and prepare vaccines. RNA vaccines can avoid integration into host cell genome which may lead to potential risks, they have less side effects and lower autoimmunity. But the possiblity of rapid degradation and clearance may lead to lower potency. Peptides are an attractive choice as vaccines due to their potential to directly function as pivotal T-cell epitopes [11]. The advantages include their low toxicity profiles, specificity for target, relatively low cost, and can be prepared by chemical synthesis. In the meanwhile, multiple peptide epitopes can be incorporate in a single vaccine to increase the chance of activating multiple T-cells and avoid tumor escape caused by loss or changes of epitopes during tumor progression [11,12,13]. The peptide vaccine tends to be the most commonly used in clinical trials.
How to develop personalized neoantigen vaccines?
Although there are some differences among the companies developing neoantigen vaccines, normally there is a general process.
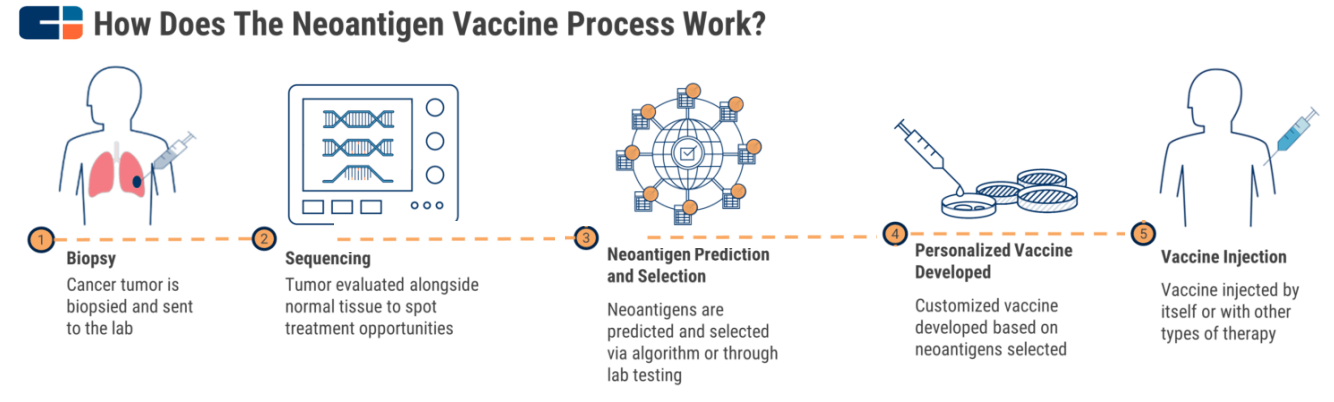
Fig.4 How neoantigen vaccines work. Source: CB Insights [14]
The first step is to select the neoantigens that will be most effective and decide whether they are public (also called "shared") or personized. Public neoantigens, are mutation-based antigens common amongst certain tumor types or repeatedly occur in many patients. For people with tumors bearing a specific public neoantigen, an off-the-shelf vaccine can be developed and administered to multiple patients [15]. However, neoantigens always differ from patient to patient, and tumor to tumor. For those patients without a public neoantigen, a vaccine needs to be purely personalized to ensure antitumor response. Thus, personalized treatment is more complex and costly. With the significantly improvement of prediction algorithms and the using of sophisticated machine learning methods, many companies are now able to improve the success rate of selecting neoantigen candidates.
Once the neoantigens are selected, the next step is to develop and manufacture the vaccine based on different strategies.
- Chemically synthesize the neoantigen peptide, then inject the purified neoantigen into the patient to elicit T-cell response.
- Synthesize the RNA or DNA as templates.
- Use a virus to infect cancer cells, lysing and spilling the neoantigens into the environment.
- In the lab, generate antigen presenting cells (APCs) from a patient's own blood and reinject it back into the patient, where the APCs will stimulate T-cell activity [15].
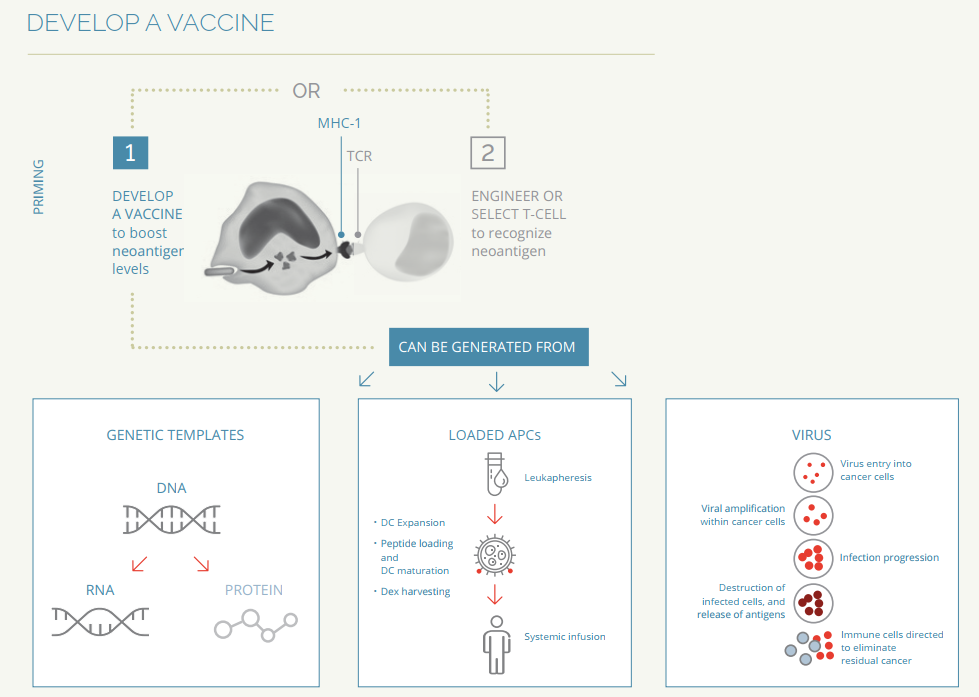
Fig.5 Multiple methods to develop vaccines. Source: BACK BAY LIFE SCIENCE ADVISORS [15].
At last when the vaccine is ready, it is administered to the patients. Comparative studies have shown that the combination of tumor vaccine and other therapies can be more effective than monotherapy. So mapping out how these neoantigen vaccines could combine with other therapies or drugs is also an important part for successful clinical response.
Strategies to improve personalized neoantigen vaccines for cancer
In the paper published by Catherine J. Wu and colleagues, they proposed four strategies that can be expected to improve the neoantigen vaccines in the near term, as illustrated in the below figure [16].
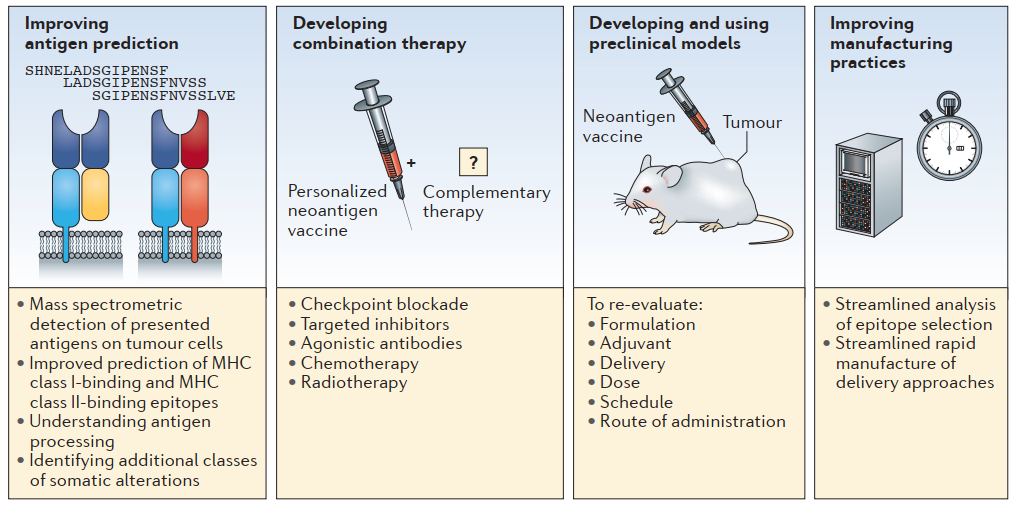
Fig.6 Strategies to improve personalized neoantigen vaccines for cancer [16]
First one, improving the antigen prediction. Improve HLA-binding algorithms to increase the possibilities of targeting neoantigens that are expressed by cancer cells and mass spectrometry-based approaches can identify peptides that are processed and presented by the tumor cell [16].
Second, developing combination therapy. Personalized neoantigen vaccines can be combined with other therapies such as checkpoint blockade to prevent immune escape. Complementary therapies to reverse immune suppression in the tumor microenvironment, such as depleting regulatory cells, inhibiting regulatory molecules or blocking metabolic suppression, will be important to unleash the full potential of a neoantigen-based cancer vaccine [16]. Combinations of neoantigen vaccine and adaptive T cell therapy have also been successfully used to achieve anti-tumor response. Traditional treatments such as radiotherapy and chemotherapy can also enhance the role of neoantigen vaccines.
Third, developing and using preclinical models. The use of preclinical models is important to optimize dosing, administration routes, immune adjuvants and vaccine delivery approaches [16].
Fourth, improving manufacturing practices. Production of personalized vaccines could be more costly and time-consuming. Streamlined analysis and selection of epitopes and streamlined rapid manufacture of peptide or DNA will substantially lower the cost and production time of personalized vaccines [16].
"Cancer immunotherapy is a therapy used to treat cancer patients that involves or uses components of the immune system. Some cancer immunotherapies consist of antibodies that bind to, and inhibit the function of, proteins expressed by cancer cells. Other cancer immunotherapies include vaccines and T cell infusions."
Nature.com
The 2018 Nobel Prize in Physiology or Medicine was awarded to James P. Allison and Tasuku Honjo for their discovery of cancer therapy by inhibition of negative immune regulation. It's not an overnight success, as harnessing the immune system to eliminate tumors have been under development since the start of 20th century. Several effective strategies emerged over the past decade are now widely considered as promising tools for the treatment of cancers. CAR-T cell therapy and immune checkpoint blockade (CPB) have been shown effective in clinical trials. Nevertheless, both CAR-T and CPB have limitations. The CAR-T approach could not work well for solid tumors, and the objective response rate of CPB is limited in most tumor types. Therefore, only a small population of patients could benefit from these therapy approaches. Increasing research then focused on understanding the biological basis, identifying which patients are likely respond or not respond to the therapies, and how to increase the effectiveness. A rational approach is to combine checkpoint blockade therapy with personalized neoantigen vaccines.
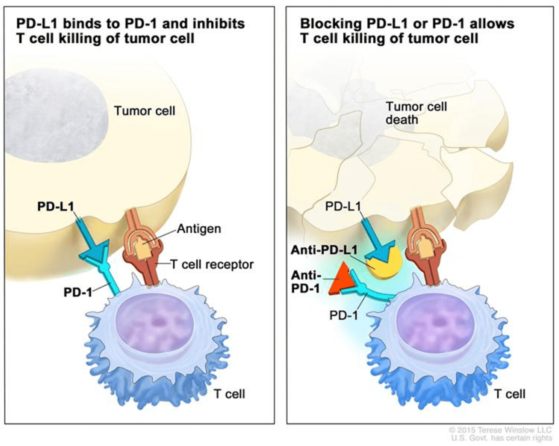
Fig.7 Blocking PD-1 or PD-L1 allows T cell killing the tumor cell. Source: National Cancer Institute

As mentioned above, two studies published in 2017 showed efficacy of neoantigen vaccines in humans. One of the studies was conducted by co-founders of Neon Therapeutics- Nir Hacohen and Catherine Wu. The other one was conducted by Ugur Sahin and colleagues from Biopharmaceutical New Technologies (BioNTech) Corporation and the Johannes Gutenberg University in Germany. Ugur Sahin is the founder & CEO of BioNTech. Neon Therapeutics and BioNTech are the leading players in the neoantigen vaccine industry. Besides them, Back Bay Life Science Advisors cataloged more than 30 companies who have laid claim to a neoantigen selection and/or priming method (see fig.9, [15]). These biopharmaceutical companies are building their own techniques and platforms to develop proprietary neoantigen vaccines and therapy methods. Below, we take a look at some of the key players in the industry.

- Peng, Miao, et al. "Neoantigen vaccine: an emerging tumor immunotherapy." Molecular cancer 18.1 (2019): 1-14.
- Lu, Yong-Chen, and Paul F. Robbins. "Cancer immunotherapy targeting neoantigens." Seminars in immunology. Vol. 28. No. 1. Academic Press, 2016.
- Jiang, Tao, et al. "Tumor neoantigens: from basic research to clinical applications." Journal of hematology & oncology 12.1 (2019): 93.
- De Plaen, Etienne, et al. "Immunogenic (tum-) variants of mouse tumor P815: cloning of the gene of tum-antigen P91A and identification of the tum-mutation." Proceedings of the National Academy of Sciences 85.7 (1988): 2274-2278.
- Matsushita, Hirokazu, et al. "Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting." Nature 482.7385 (2012): 400-404.
- Castle, John C., et al. "Exploiting the mutanome for tumor vaccination." Cancer research 72.5 (2012): 1081-1091.
- Editorial, N. B. "The problem with neoantigen prediction." Nat Biotechnol 35 (2017): 97.
- Gros, Alena, Andrea Garcia-Garijo, and Carlos Alberto Fajardo. "Determinants for neoantigen identification." Frontiers in Immunology 10 (2019): 1392.
- Carreno, Beatriz M., et al. "A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells." Science 348.6236 (2015): 803-808.
- Chu, Yanhong, et al. "Personalized cancer neoantigen vaccines come of age." Theranostics 8.15 (2018): 4238.
- Aldous, Amanda R., and Jesse Z. Dong. "Personalized neoantigen vaccines: A new approach to cancer immunotherapy." Bioorganic & medicinal chemistry 26.10 (2018): 2842-2849.
- Mullard, Asher. "The cancer vaccine resurgence." (2016): 663.
- Kumai, Takumi, et al. "Peptide vaccines in cancer—old concept revisited." Current opinion in immunology 45 (2017): 1-7.
- CB Insights, 2019. “New Personalized Cancer Treatment report”
- BACK BAY LIFE SCIENCE ADVISORS, JUNE 1, 2018. “Neoantigen-based cancer immunotherapy: one step closer to the promise of personalized medicine.”
- Hu, Zhuting, Patrick A. Ott, and Catherine J. Wu. "Towards personalized, tumour-specific, therapeutic vaccines for cancer." Nature Reviews Immunology 18.3 (2018): 168.
- Ott, Patrick A., et al. "An immunogenic personal neoantigen vaccine for patients with melanoma." Nature 547.7662 (2017): 217-221.
- Sahin, Ugur, et al. "Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer." Nature 547.7662 (2017): 222-226.
GenScript provides a full spectrum of high-quality peptide services ranging from Custom Peptide Synthesis (research and cGMP grade with Regular and Express deliveries) to high-throughput Peptide Library and Peptide Array services. See how GenScript can facilitate your neoantigen research ?
Related Resource
- GenScript Peptide Service Portfolio
- GenScript Peptide Promotion
- Peptide Applications -Cancer Immunotherapy
- Peptide Resource Center
Limited-time Campaign
